Time:2024-12-05
Formation and Properties of Nitroso Compounds
Amines, amides, amino esters, urea, and guanidine compounds react with nitrites or other nitrogen-containing reagents (such as N₂O₃and N₂O₄) to form nitroso compounds.
The International Agency for Research on Cancer (IARC) has classified various nitroso compounds as Group 2A or Group 2B carcinogens (i.e. potential or potential carcinogens) based on a large amount of research data. The International Coordinating Committee (ICH) M7 guidelines also list it as a 'Group of Substances of Concern'.

Figure 1: Common types and structural characteristics of Nitroso compounds
In recent years, nitroso compounds (especially nitrosamines) have attracted high attention from the global pharmaceutical industry and regulatory agencies. The cause of this phenomenon is the detection of trace amounts of N-nitrosodimethylamine (NDMA) in angiotensin II receptor antagonists (ARB drugs, such as valsartan, losartan, irbesartan, and olmesartan). In 2019, NDMA was also detected in metformin and ranitidine. Since then, drug recalls caused by the detection of other types of nitrosamine impurities have become increasingly common, such as rifampicin, rifampicin, varenicline, quinapril, and hydrochlorothiazide. Regulatory agencies around the world have released multiple guidance documents and recommendations to address the risk of nitrosamine impurities in drugs.
It is worth noting that in practical work, the number of nitrosamines is much larger than that of nitrosamides, which often blurs the boundary between "nitrosamines" and "nitrosamides". This leads to researchers mistakenly equating nitrosamides with nitrosamines in risk assessments and adopting incorrect research methods. But if you carefully study the official documents, you can find that the more accurate term Nitros - is often used in regulatory guidelines to refer to the risk of "nitroso" compounds.
From an organic chemistry perspective, nitrosamides (such as amides, N-alkylureas, guanidine derivatives, or nitroso derivatives of amino esters) are structurally and qualitatively distinct from nitroso derivatives of amines (i.e. nitrosamines). Nitrosamides have high chemical reactivity and can directly interact with nucleophilic substrates and free radicals, exhibiting mutagenicity or carcinogenicity without the need for metabolic activation. However, most nitrosamides have high chemical reactivity, and even if they are produced, they will quickly degrade into other impurities that cannot be separated; However, as a comparison, nitrosamines have good chemical stability. Metabolized nitrosamines are less likely to interact with biomolecules or other chemical molecules and will remain stable in drugs until they are activated through metabolism before they can take effect. Therefore, these two types of compounds should not be confused.
(Pharmaceutical Technology, Trends in Formulation, 2022 eBook, 42–50.)
Discussion: Differences in activation mechanisms between Nitrosamides and Nitrosamines
Nitrosamines and nitrosamides are the two main types of structures in nitroso compounds. Nitrosamine is formed by the reaction of secondary or tertiary amines with nitrite or other nitrosating agents, and its chemical structure can be considered as an "amine derivative" of nitrite. Its metabolism mainly relies on the α-hydroxylation catalyzed by cytochrome P450, which produces positively charged electrophilic compounds, thereby causing alkylation damage to DNA (Figure 2). Therefore, nitrosamines are usually stable in vitro and widely present in the environment and drugs. The carcinogenicity of nitrosamines needs to be demonstrated through metabolic activation.
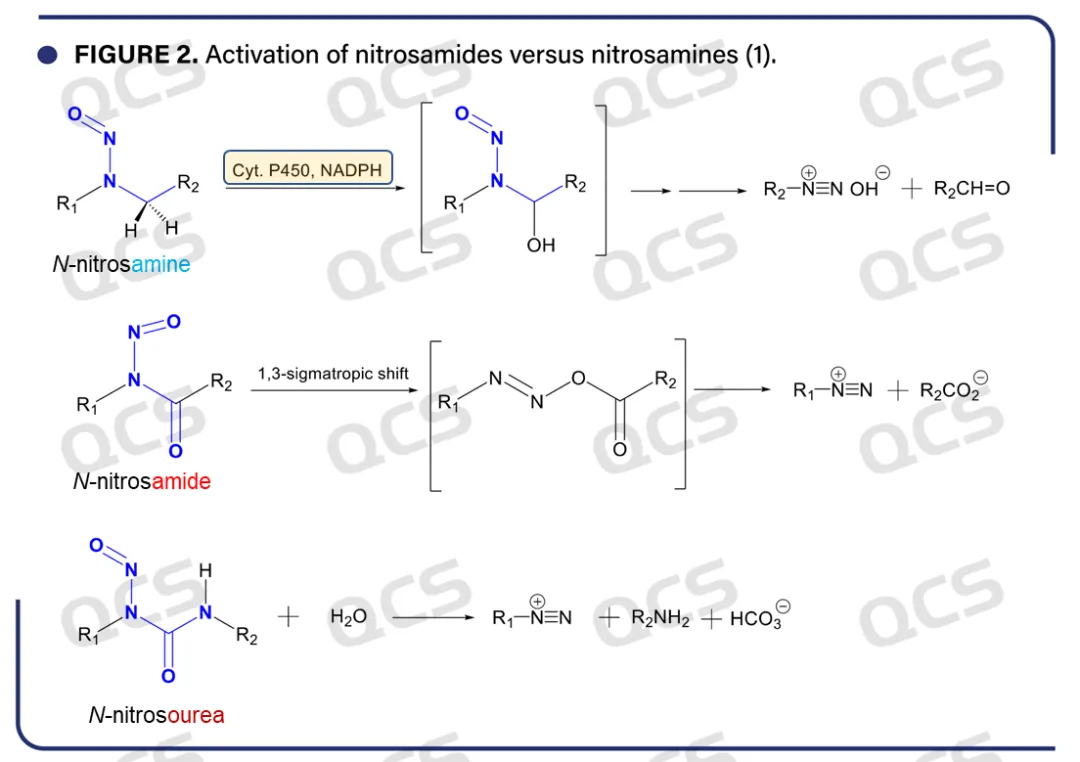
Figure 2: Metabolic pathways of mutagenic compounds such as nitrosamines and nitrosamides
In contrast, nitrosamides (such as N-nitrosamides, N-nitroso amino acid esters, N-nitroso urea, and N-nitroso guanidine compounds) are significantly different in chemical structure and activation mechanism from nitrosamines. In the structure of nitrosaminds, the synergistic effect between nitroso (- NO) and carbonyl (C=O or C=N) allows for the direct generation of electrophilic compounds without metabolic activation. This direct mode of action gives nitrosamides higher mutagenicity than nitrosamines, but also brings significant chemical instability.
Although we can see that some nitrosamide compounds (such as Camustine and Lomustine) exhibit anti-cancer potential and are used as active ingredients in anti-tumor drugs, most nitrosamides have poor stability under drug production or storage conditions and can rapidly degrade with nucleophilic reagents such as water molecules (Figure 3, ACS Symposium Series, 1993, 553, 1-18).
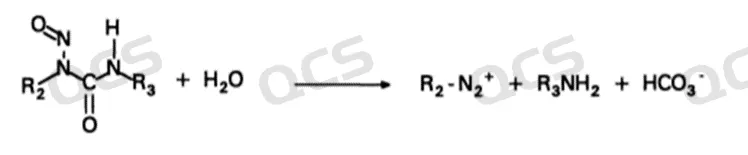
Figure 3: Reaction between Nitrosamides and Water Molecules
Nitrosamides may undergo rapid decomposition reactions even without the addition of any exogenous reagents. Referring to the results of researchers Julius Rebek Jr. and Fahmi Himo et(Reference: Tetr Lett 2011, 52, 2100-2103; J. Org. Chem. 2019, 84, 7354-7361), it was shown that N-butyl-4-methyl-N-nitrosobenzoamide would decompose significantly within one day at 35 ℃, until it was completely decomposed into the corresponding ester compound after 67 hours. If it is a nitroso derivative of a first-order amide, it can even be degraded into the corresponding carboxylic acid product (J. Am. Chem. Soc. 2002, 124, 14115-14126). Experiments have shown that this degradation pathway is widely present under common storage conditions, highlighting the instability of Nitrosamides.
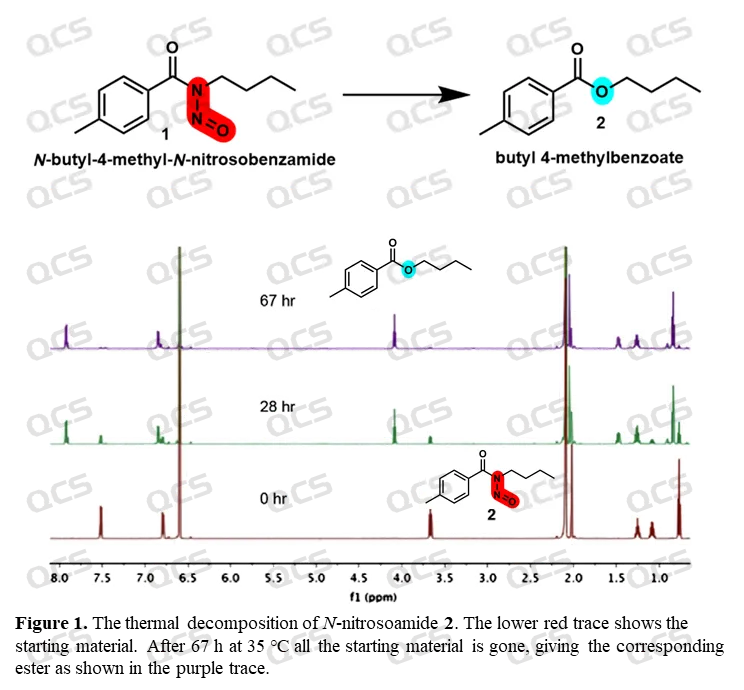
Figure 4: Monitoring of self degradation reaction of N-butyl-4-methyl-N-nitrosobenzoamide
In addition, other degradation reactions may occur due to environmental factors such as light or acidity (Can. J. Chem. 1969, 47, 2441-2448). For example, under light conditions, Nitrosamides may quickly decompose to produce amides or rearrangement products, which can be completed in a few hours at room temperature. Similar degradation reactions also occur in nitrososulfonamide structures(Recl. Trav. Chim. Pays‐Bas 1971, 90, 901–905.)

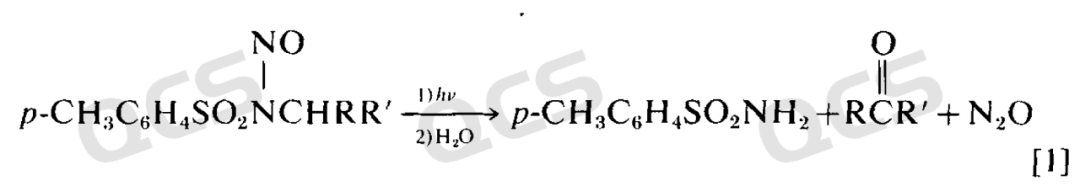
Figure 5: Nitrosamides decomposition products
These degradation reactions can refer to the following reaction mechanisms.
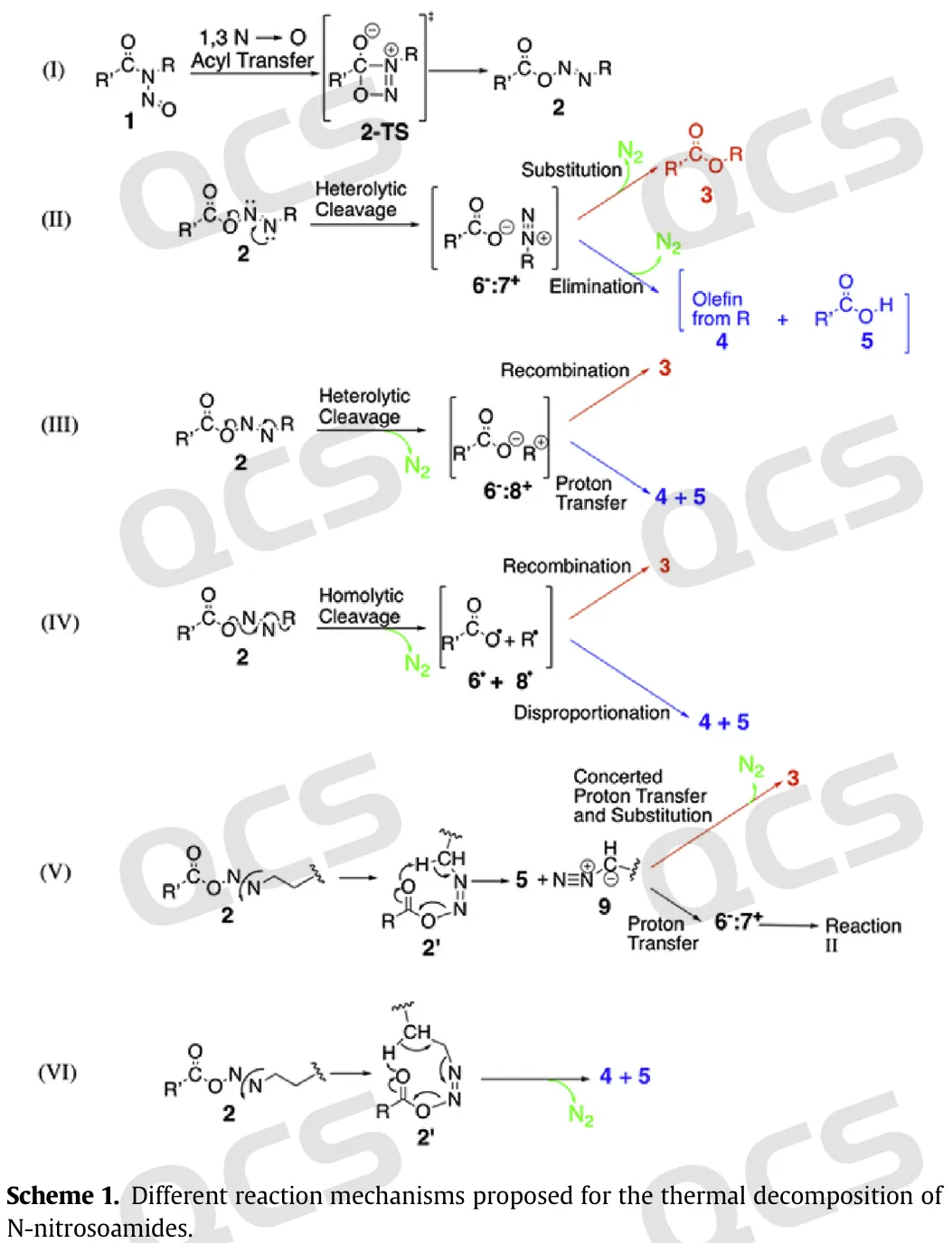
Figure 6: Self degradation mechanism of Nitrosamides
Summary
Given the significant differences in activation mechanism, stability, and toxic effects between Nitrosamines and Nitrosamides, they should not be treated equally in risk assessment. In most cases, even if there is a risk of producing Nitrosamide impurities, the corresponding Nitrosamide often cannot exist stably in the material and will degrade into other impurities in a very short period of time. Therefore, for the risk assessment of Nitrosamides, the first consideration should be whether the Nitrosamide impurities in the raw material or related substances are stably present in the material, and then a reasonable method should be selected for qualitative and quantitative testing.
At the same time, we should also note that more and more regulatory agencies in various countries are beginning to clearly distinguish the risks of Ntrosamines and Nitrosamides in their guidance documents to avoid confusion in assessment or regulation.

Formation and Properties of Nitroso Compounds
Amines, amides, amino esters, urea, and guanidine compounds react with nitrites or other nitrogen-containing reagents (such as N₂O₃and N₂O₄) to form nitroso compounds.
The International Agency for Research on Cancer (IARC) has classified various nitroso compounds as Group 2A or Group 2B carcinogens (i.e. potential or potential carcinogens) based on a large amount of research data. The International Coordinating Committee (ICH) M7 guidelines also list it as a 'Group of Substances of Concern'.

Figure 1: Common types and structural characteristics of Nitroso compounds
In recent years, nitroso compounds (especially nitrosamines) have attracted high attention from the global pharmaceutical industry and regulatory agencies. The cause of this phenomenon is the detection of trace amounts of N-nitrosodimethylamine (NDMA) in angiotensin II receptor antagonists (ARB drugs, such as valsartan, losartan, irbesartan, and olmesartan). In 2019, NDMA was also detected in metformin and ranitidine. Since then, drug recalls caused by the detection of other types of nitrosamine impurities have become increasingly common, such as rifampicin, rifampicin, varenicline, quinapril, and hydrochlorothiazide. Regulatory agencies around the world have released multiple guidance documents and recommendations to address the risk of nitrosamine impurities in drugs.
It is worth noting that in practical work, the number of nitrosamines is much larger than that of nitrosamides, which often blurs the boundary between "nitrosamines" and "nitrosamides". This leads to researchers mistakenly equating nitrosamides with nitrosamines in risk assessments and adopting incorrect research methods. But if you carefully study the official documents, you can find that the more accurate term Nitros - is often used in regulatory guidelines to refer to the risk of "nitroso" compounds.
From an organic chemistry perspective, nitrosamides (such as amides, N-alkylureas, guanidine derivatives, or nitroso derivatives of amino esters) are structurally and qualitatively distinct from nitroso derivatives of amines (i.e. nitrosamines). Nitrosamides have high chemical reactivity and can directly interact with nucleophilic substrates and free radicals, exhibiting mutagenicity or carcinogenicity without the need for metabolic activation. However, most nitrosamides have high chemical reactivity, and even if they are produced, they will quickly degrade into other impurities that cannot be separated; However, as a comparison, nitrosamines have good chemical stability. Metabolized nitrosamines are less likely to interact with biomolecules or other chemical molecules and will remain stable in drugs until they are activated through metabolism before they can take effect. Therefore, these two types of compounds should not be confused.
(Pharmaceutical Technology, Trends in Formulation, 2022 eBook, 42–50.)
Discussion: Differences in activation mechanisms between Nitrosamides and Nitrosamines
Nitrosamines and nitrosamides are the two main types of structures in nitroso compounds. Nitrosamine is formed by the reaction of secondary or tertiary amines with nitrite or other nitrosating agents, and its chemical structure can be considered as an "amine derivative" of nitrite. Its metabolism mainly relies on the α-hydroxylation catalyzed by cytochrome P450, which produces positively charged electrophilic compounds, thereby causing alkylation damage to DNA (Figure 2). Therefore, nitrosamines are usually stable in vitro and widely present in the environment and drugs. The carcinogenicity of nitrosamines needs to be demonstrated through metabolic activation.

Figure 2: Metabolic pathways of mutagenic compounds such as nitrosamines and nitrosamides
In contrast, nitrosamides (such as N-nitrosamides, N-nitroso amino acid esters, N-nitroso urea, and N-nitroso guanidine compounds) are significantly different in chemical structure and activation mechanism from nitrosamines. In the structure of nitrosaminds, the synergistic effect between nitroso (- NO) and carbonyl (C=O or C=N) allows for the direct generation of electrophilic compounds without metabolic activation. This direct mode of action gives nitrosamides higher mutagenicity than nitrosamines, but also brings significant chemical instability.
Although we can see that some nitrosamide compounds (such as Camustine and Lomustine) exhibit anti-cancer potential and are used as active ingredients in anti-tumor drugs, most nitrosamides have poor stability under drug production or storage conditions and can rapidly degrade with nucleophilic reagents such as water molecules (Figure 3, ACS Symposium Series, 1993, 553, 1-18).

Figure 3: Reaction between Nitrosamides and Water Molecules
Nitrosamides may undergo rapid decomposition reactions even without the addition of any exogenous reagents. Referring to the results of researchers Julius Rebek Jr. and Fahmi Himo et(Reference: Tetr Lett 2011, 52, 2100-2103; J. Org. Chem. 2019, 84, 7354-7361), it was shown that N-butyl-4-methyl-N-nitrosobenzoamide would decompose significantly within one day at 35 ℃, until it was completely decomposed into the corresponding ester compound after 67 hours. If it is a nitroso derivative of a first-order amide, it can even be degraded into the corresponding carboxylic acid product (J. Am. Chem. Soc. 2002, 124, 14115-14126). Experiments have shown that this degradation pathway is widely present under common storage conditions, highlighting the instability of Nitrosamides.

Figure 4: Monitoring of self degradation reaction of N-butyl-4-methyl-N-nitrosobenzoamide
In addition, other degradation reactions may occur due to environmental factors such as light or acidity (Can. J. Chem. 1969, 47, 2441-2448). For example, under light conditions, Nitrosamides may quickly decompose to produce amides or rearrangement products, which can be completed in a few hours at room temperature. Similar degradation reactions also occur in nitrososulfonamide structures(Recl. Trav. Chim. Pays‐Bas 1971, 90, 901–905.)

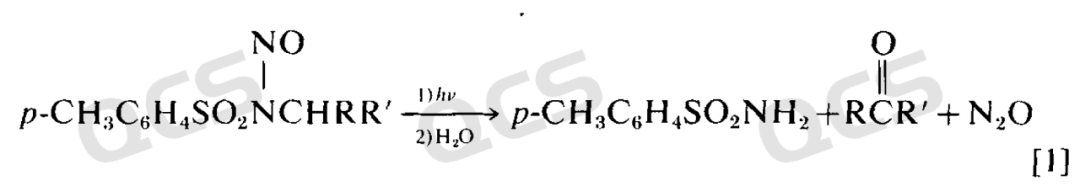
Figure 5: Nitrosamides decomposition products
These degradation reactions can refer to the following reaction mechanisms.

Figure 6: Self degradation mechanism of Nitrosamides
Summary
Given the significant differences in activation mechanism, stability, and toxic effects between Nitrosamines and Nitrosamides, they should not be treated equally in risk assessment. In most cases, even if there is a risk of producing Nitrosamide impurities, the corresponding Nitrosamide often cannot exist stably in the material and will degrade into other impurities in a very short period of time. Therefore, for the risk assessment of Nitrosamides, the first consideration should be whether the Nitrosamide impurities in the raw material or related substances are stably present in the material, and then a reasonable method should be selected for qualitative and quantitative testing.
At the same time, we should also note that more and more regulatory agencies in various countries are beginning to clearly distinguish the risks of Ntrosamines and Nitrosamides in their guidance documents to avoid confusion in assessment or regulation.

Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号