Time:2022-07-21
An increasing number of reference product manufacturers are including thermal analysis data in their quality control reports, with TGA (Thermogravimetric Analysis) being the most commonly reported method. What exactly is TGA?
Thermogravimetric Analysis (TGA) measures changes in the mass of a sample as a function of temperature or time, within a controlled temperature range and under specified conditions.
By analyzing the thermogravimetric (TG) curve, we can determine the thermal stability of the sample, identify its thermal decomposition behavior, and assess impurity composition as well as other quality-related characteristics. The key features of thermogravimetric analysis include its broad applicability, minimal sample consumption, high sensitivity, and precise measurement of mass changes and their rates. Essentially, any substance that exhibits mass loss upon heating due to volatile component release can be effectively studied using thermogravimetric analysis.
The subsequent TGA report, provided by QCS manufacturer (available at https://www.qcsrm.com), serves as an illustrative example to elucidate the interpretation of TGA data.
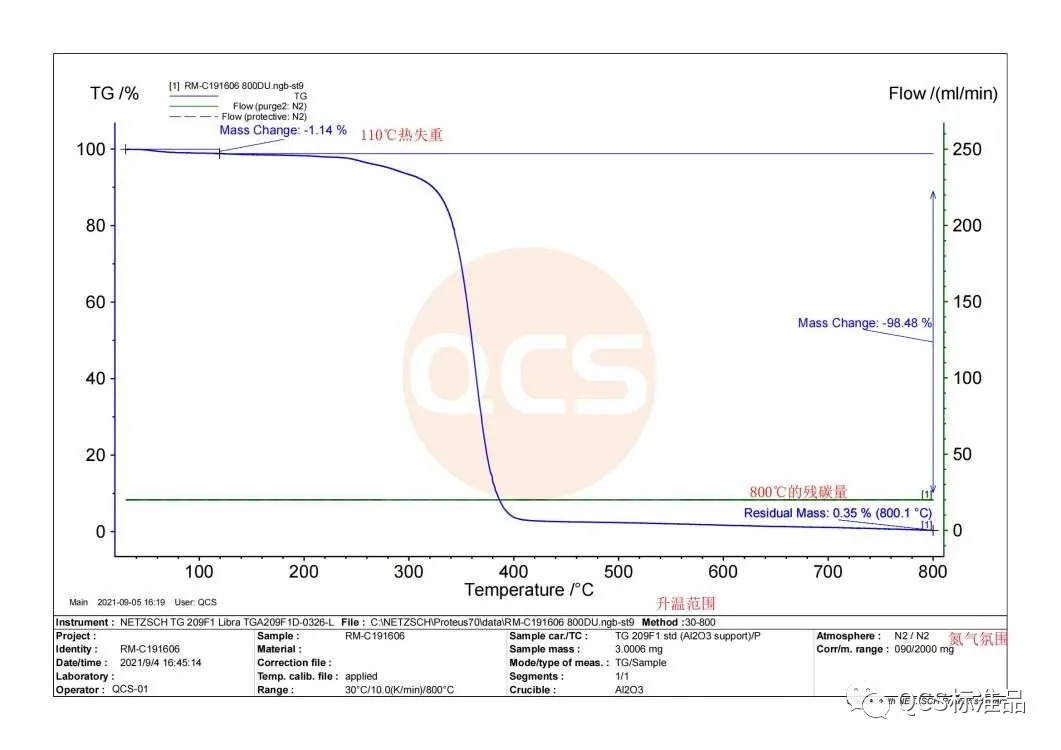
As illustrated in the figure, QCS manufacturer utilize the TGA equipment TG209F1 from Netzsch to analyze cyclosporin impurity A products under a nitrogen atmosphere with a temperature range of 30-800 °C. The results indicate that within the 30-110 °C range, the product experiences a mass loss of 1.14%. Between 110 and 400 °C, the mass loss exceeds 99%, indicating significant thermal degradation. In the 400-600 °C range, the mass loss is minimal and tends toward equilibrium, suggesting the completion of thermal decomposition. Finally, at 800 °C, the residual carbon content is 0.35%.The data reflects the changes in product quality within a specific temperature range. But what is the meaning of these data, I propose we discuss them in detail.
Stage 1 (30-105 °C): The gentle weight loss curve, primarily due to desorption of adsorbed water, results in a 1.14% mass loss. This stage typically involves the loss of crystal water and bound water around 100-120 °C. If the sample contains a higher proportion of low-boiling solvent residues, a pronounced weight loss peak may be observed. However, the absence of an evident weight loss peak in this figure suggests that either the amount of residual solvent is minimal or its volatility is comparable to that of water.
Stage 2 (110-400 °C): The sharp change in the weight loss curve starting around 300 °C indicates the onset of thermal degradation, consistent with the decomposition of most organic materials between 250-300 °C.
Stage 3 (400-600 °C): Minimal mass loss suggests that thermal decomposition is largely complete, leading to stabilization of the sample's mass.
Stage 4 (800 °C): The residual carbon content of 0.35% cannot be equated directly with ash content due to the nitrogen atmosphere used. Due to the employment of a nitrogen atmosphere, carbon residue is present in the remaining material. In contrast, if an air atmosphere is utilized, the residual material would be classified as ash. These two conditions yield distinct outcomes.
For impurity calibration, it is essential to measure chromatographic purity, dry weight loss, and incandescent residue. However, given the limited sample quantity, this method is frequently employed as a substitute for combustion residue analysis, with due consideration given to the inherent differences. Additionally, some manufacturers exclusively employ thermogravimetric analysis (TGA) to characterize moisture, soluble residues, and residual materials. Specifically, they measure the mass loss percentage at 105°C to represent the combined content of moisture and soluble residues, and subsequently determine the ash content based on the residual mass at 800°C. Finally, these measurements are converted into the respective contents. While this method is convenient, it lacks supporting data from other tests, posing potential risks.
Finally, we present two advanced integrated thermal analysis techniques. Simultaneous Thermal Analysis (STA) integrates Thermogravimetric Analysis (TGA) with Differential Thermal Analysis (DTA) or Differential Scanning Calorimetry (DSC), thereby obtaining thermogravimetric and differential thermal data concurrently from the same sample during a single measurement. Additionally, Thermogravimetric Analysis coupled with Gas Chromatography and Mass Spectrometry (TGA-GC-MS) enables the acquisition of thermogravimetric curves while simultaneously analyzing volatile components through GC-MS.

An increasing number of reference product manufacturers are including thermal analysis data in their quality control reports, with TGA (Thermogravimetric Analysis) being the most commonly reported method. What exactly is TGA?
Thermogravimetric Analysis (TGA) measures changes in the mass of a sample as a function of temperature or time, within a controlled temperature range and under specified conditions.
By analyzing the thermogravimetric (TG) curve, we can determine the thermal stability of the sample, identify its thermal decomposition behavior, and assess impurity composition as well as other quality-related characteristics. The key features of thermogravimetric analysis include its broad applicability, minimal sample consumption, high sensitivity, and precise measurement of mass changes and their rates. Essentially, any substance that exhibits mass loss upon heating due to volatile component release can be effectively studied using thermogravimetric analysis.
The subsequent TGA report, provided by QCS manufacturer (available at https://www.qcsrm.com), serves as an illustrative example to elucidate the interpretation of TGA data.

As illustrated in the figure, QCS manufacturer utilize the TGA equipment TG209F1 from Netzsch to analyze cyclosporin impurity A products under a nitrogen atmosphere with a temperature range of 30-800 °C. The results indicate that within the 30-110 °C range, the product experiences a mass loss of 1.14%. Between 110 and 400 °C, the mass loss exceeds 99%, indicating significant thermal degradation. In the 400-600 °C range, the mass loss is minimal and tends toward equilibrium, suggesting the completion of thermal decomposition. Finally, at 800 °C, the residual carbon content is 0.35%.The data reflects the changes in product quality within a specific temperature range. But what is the meaning of these data, I propose we discuss them in detail.
Stage 1 (30-105 °C): The gentle weight loss curve, primarily due to desorption of adsorbed water, results in a 1.14% mass loss. This stage typically involves the loss of crystal water and bound water around 100-120 °C. If the sample contains a higher proportion of low-boiling solvent residues, a pronounced weight loss peak may be observed. However, the absence of an evident weight loss peak in this figure suggests that either the amount of residual solvent is minimal or its volatility is comparable to that of water.
Stage 2 (110-400 °C): The sharp change in the weight loss curve starting around 300 °C indicates the onset of thermal degradation, consistent with the decomposition of most organic materials between 250-300 °C.
Stage 3 (400-600 °C): Minimal mass loss suggests that thermal decomposition is largely complete, leading to stabilization of the sample's mass.
Stage 4 (800 °C): The residual carbon content of 0.35% cannot be equated directly with ash content due to the nitrogen atmosphere used. Due to the employment of a nitrogen atmosphere, carbon residue is present in the remaining material. In contrast, if an air atmosphere is utilized, the residual material would be classified as ash. These two conditions yield distinct outcomes.
For impurity calibration, it is essential to measure chromatographic purity, dry weight loss, and incandescent residue. However, given the limited sample quantity, this method is frequently employed as a substitute for combustion residue analysis, with due consideration given to the inherent differences. Additionally, some manufacturers exclusively employ thermogravimetric analysis (TGA) to characterize moisture, soluble residues, and residual materials. Specifically, they measure the mass loss percentage at 105°C to represent the combined content of moisture and soluble residues, and subsequently determine the ash content based on the residual mass at 800°C. Finally, these measurements are converted into the respective contents. While this method is convenient, it lacks supporting data from other tests, posing potential risks.
Finally, we present two advanced integrated thermal analysis techniques. Simultaneous Thermal Analysis (STA) integrates Thermogravimetric Analysis (TGA) with Differential Thermal Analysis (DTA) or Differential Scanning Calorimetry (DSC), thereby obtaining thermogravimetric and differential thermal data concurrently from the same sample during a single measurement. Additionally, Thermogravimetric Analysis coupled with Gas Chromatography and Mass Spectrometry (TGA-GC-MS) enables the acquisition of thermogravimetric curves while simultaneously analyzing volatile components through GC-MS.

Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号